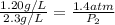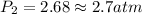Chemistry, 29.07.2019 13:00 ashleyann3052
To increase the solubility of a gas at constant temperature from 1.20 g/l, at 1.4 atm, to 2.3 g/l, the pressure would have to be increased to 2.7 atm 0.7 atm 0.37 atm 1.37 atm

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1


Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
To increase the solubility of a gas at constant temperature from 1.20 g/l, at 1.4 atm, to 2.3 g/l, t...
Questions in other subjects:

Mathematics, 14.11.2020 04:20








Mathematics, 14.11.2020 04:20

English, 14.11.2020 04:20

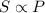

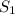 = initial solubility of gas = 1.20 g/L
= initial solubility of gas = 1.20 g/L
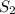 = final solubility of gas = 2.3 g/L
= final solubility of gas = 2.3 g/L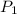 = initial pressure of gas = 1.4 am
= initial pressure of gas = 1.4 am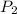 = final pressure of gas = ?
= final pressure of gas = ?