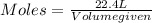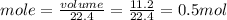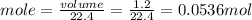Chemistry, 29.07.2019 14:00 brycehelmke60811
The molar volume of a gas at stp, in liters, is . you can use the molar volume to convert 2 mol of any gas to l. you can also use the molar volume to convert 11.2 l of any gas to mol. avogadro’s law tells you that 1.2 l of o2(g) and 1.2 l of no2(g) are of moles of gas.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
The molar volume of a gas at stp, in liters, is . you can use the molar volume to convert 2 mol of...
Questions in other subjects:

Mathematics, 07.03.2021 16:20

Computers and Technology, 07.03.2021 16:20

Chemistry, 07.03.2021 16:20



English, 07.03.2021 16:20


Mathematics, 07.03.2021 16:30

Biology, 07.03.2021 16:30

Social Studies, 07.03.2021 16:30