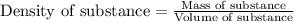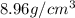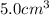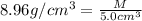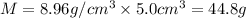Chemistry, 29.07.2019 14:30 lukecarroll19521
What is the mass of 5.0 cm^3 piece of copper having a density of 8.96 g/cm^3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 01:20, hflores0001
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 16:00, isabel81ie
Aconversion factor is a ratio of equivalent values used to express the same units in different quantities
Answers: 3
You know the right answer?
What is the mass of 5.0 cm^3 piece of copper having a density of 8.96 g/cm^3...
Questions in other subjects:



Biology, 27.02.2020 01:19

Computers and Technology, 27.02.2020 01:19


Physics, 27.02.2020 01:19

Mathematics, 27.02.2020 01:19

Physics, 27.02.2020 01:19