
Chemistry, 29.07.2019 16:30 julieariscar769
Assuming that the density of vinegar is 1.005 g/ml, calculate the molarity of acetic acid in vinegar from your average value for the mass percentage of acetic acid in vinegar. density of vinegar= 1.005 g/ml average mass % acetic acid= 5.2%,

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 02:30, elyzarobertson
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Assuming that the density of vinegar is 1.005 g/ml, calculate the molarity of acetic acid in vinegar...
Questions in other subjects:

History, 26.05.2021 15:50


English, 26.05.2021 15:50

Mathematics, 26.05.2021 15:50




Mathematics, 26.05.2021 15:50

Geography, 26.05.2021 15:50

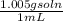 =1005 g soln
=1005 g soln

