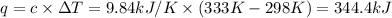The combustion of titanium with oxygen produces titanium dioxide: ti (s) + o2(g) â tio2 (s) when 1.000 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 â°c to 60.00 â°c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
The combustion of titanium with oxygen produces titanium dioxide: ti (s) + o2(g) â tio2 (s) when 1....
Questions in other subjects:

Mathematics, 21.05.2021 20:30



Mathematics, 21.05.2021 20:30

Chemistry, 21.05.2021 20:30


Mathematics, 21.05.2021 20:30



Social Studies, 21.05.2021 20:30