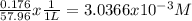Chemistry, 30.07.2019 01:30 Alexandragurule18
Calculate the external pressure that must be applied to seawater, 1.14 m total ion concentration at 10 degrees c if the maximum concentration allowed in the product water is 176 mg/l. assume that all the dissolved salts in the product water is sodium chloride. so, i know that i need to subtract the ion concentrations before using the pi=mrt formula, but i can't figure out how to convert mg/l into molarity. !

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Calculate the external pressure that must be applied to seawater, 1.14 m total ion concentration at...
Questions in other subjects:

English, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Social Studies, 14.09.2020 16:01

History, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Social Studies, 14.09.2020 16:01