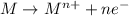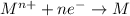Chemistry, 30.07.2019 13:30 onlymyworld27
When an atom in a reactant loses electrons, what happens to its oxidation number? a) its oxidation number decreases b) its oxidation number doubles c) its oxidation number increases d) its oxidation number stays the same

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
When an atom in a reactant loses electrons, what happens to its oxidation number? a) its oxidation...
Questions in other subjects:



Mathematics, 08.11.2019 17:31

Business, 08.11.2019 17:31



Computers and Technology, 08.11.2019 17:31