
Chemistry, 30.07.2019 15:30 jacobdobson5856
Why does water (h2o) have a significantly higher boiling point than methane (ch4)? hydrogen forms a stronger covalent bond with oxygen than with carbon. hydrogen forms a weaker covalent bond with oxygen than with carbon. water molecules are attracted to one another by hydrogen bonds. water molecules are made of fewer atoms than methane molecules.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Why does water (h2o) have a significantly higher boiling point than methane (ch4)? hydrogen forms a...
Questions in other subjects:


Social Studies, 18.10.2020 04:01

History, 18.10.2020 04:01



Mathematics, 18.10.2020 04:01


English, 18.10.2020 04:01

Mathematics, 18.10.2020 04:01

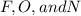 ) and the lone pair electrons present in the other molecule on these electronegative atoms is known as hydrogen bonding.
) and the lone pair electrons present in the other molecule on these electronegative atoms is known as hydrogen bonding.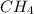 is Van der Waals interactions whereas in water,
is Van der Waals interactions whereas in water, 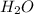 is hydrogen bonding.
is hydrogen bonding.

