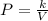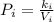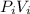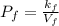Chemistry, 31.07.2019 06:30 zlittleton2008
Acertain gas is present in a 12.0 l cylinder at 4.0 atm pressure. if the pressure is increased to 8.0 atm the volume of the gas decreases to 6.0 l . find the two constants ki, the initial value of k, and kf, the final value of k, to verify whether the gas obeys boyle’s law by entering the numerical values for ki and kf in the space provided.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Acertain gas is present in a 12.0 l cylinder at 4.0 atm pressure. if the pressure is increased to 8....
Questions in other subjects:

English, 17.08.2020 02:01

Mathematics, 17.08.2020 02:01

Geography, 17.08.2020 02:01


English, 17.08.2020 02:01

Mathematics, 17.08.2020 02:01

History, 17.08.2020 02:01


Chemistry, 17.08.2020 02:01

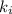
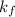
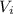 = 12.0 L
= 12.0 L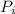 = 4.0 atm
= 4.0 atm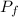 = 8.0 atm
= 8.0 atm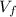 = 6.0 L
= 6.0 L