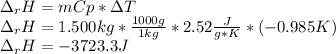Chemistry, 31.07.2019 12:00 anabelleacunamu
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbon dioxide (co2), and water (h2o). a chemist carries out this reaction in a bomb calorimeter. the reaction causes the temperature of a bomb calorimeter to decrease by 0.985 k. the calorimeter has a mass of 1.500 kg and a specific heat of 2.52 j/g•k. what is the heat of reaction for this system?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 23.06.2019 09:20, weridness80
Which of the following occurs along coasts during the day?
Answers: 3

Chemistry, 23.06.2019 13:30, swagjlove32
Asap a 50.0 ml soap bubble is blown in a 27.0°c room. it drifts out an open window and lands in a snow bank at -3.0°c. what is its new volume?
Answers: 1
You know the right answer?
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbo...
Questions in other subjects:



Chemistry, 22.10.2020 02:01




Computers and Technology, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01