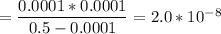Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2


Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Calculate the acid dissociation constant of a weak monoprotic acid if a 0.5m solution of this acid g...
Questions in other subjects:

History, 18.08.2019 19:00


History, 18.08.2019 19:00



Mathematics, 18.08.2019 19:00

Mathematics, 18.08.2019 19:00

Mathematics, 18.08.2019 19:00


Mathematics, 18.08.2019 19:00

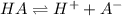
![K_a= \dfrac{[H^+][A^-]}{[HA]}](/tpl/images/0155/5739/a4583.png)
![0.5M=[A^-]+[HA]](/tpl/images/0155/5739/14b10.png)
![[HA]=0.5M-[A^-]](/tpl/images/0155/5739/ce8c0.png)
![K_a= \dfrac{[H^+][A^-]}{0.5M-[A^-]}](/tpl/images/0155/5739/a476b.png)