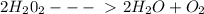Chemistry, 01.08.2019 00:30 justritegrl
In the reaction below, hydrogen peroxide decomposes to water. mm h2o2 = 34.02 g/mol mm h2o = 18.02 g/mol mm o2 = 32 g/mol 2h2o2 → 2h2o + o2 if 14.3 moles of h2o2 is decomposed, how many grams of oxygen gas are produced? show all your work to get full credit.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
In the reaction below, hydrogen peroxide decomposes to water. mm h2o2 = 34.02 g/mol mm h2o = 18.02 g...
Questions in other subjects:



Mathematics, 21.09.2020 15:01



English, 21.09.2020 15:01


History, 21.09.2020 15:01


Mathematics, 21.09.2020 15:01