Chemistry, 01.08.2019 12:00 mcclendoncassandra
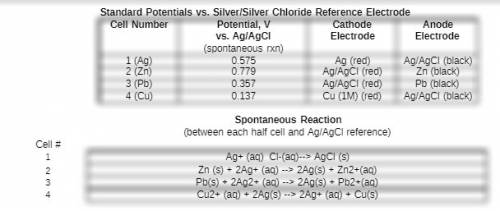
Based on your measured potential for this cell and the literature value for the standard reduction potential for the ag/agcl reference electrode, what would you expect the overall potential to be for the spontaneous reaction between your cu2+/cu electrode and a standard hydrogen eletrode? type your calculation for the expected standard reduction potential vs the she as well as the % error between this value and the literature value.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
Based on your measured potential for this cell and the literature value for the standard reduction p...
Questions in other subjects:


Mathematics, 05.02.2022 04:50

Mathematics, 05.02.2022 04:50

Mathematics, 05.02.2022 04:50


Mathematics, 05.02.2022 04:50


Mathematics, 05.02.2022 04:50

Chemistry, 05.02.2022 04:50