
Chemistry, 01.08.2019 16:00 KHaire6856
Amy measured the tire pressure of her truck that was parked outside after a cold night. the daytime temperature reached a nice 65 °f. assume amy's tires do not have any leaks. explain the change in tire pressure. 6 a. m. - 30 psi 9 a. m. - 32 psi 1 p. m. - 35 psi a) the air molecules heated inside the tire causing them to move faster exerting more pressure on the inside walls of the tires. b) the air molecules heated inside the tire causing them to move faster exerting less pressure on the inside walls of the tires. c) the air molecules heated inside the tire causing them to move slower exerting more pressure on the inside walls of the tires. d) the air molecules heated inside the tire causing them to move slower exerting less pressure on the inside walls of the tires.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Amy measured the tire pressure of her truck that was parked outside after a cold night. the daytime...
Questions in other subjects:

Mathematics, 22.02.2021 08:30




Mathematics, 22.02.2021 08:30

Mathematics, 22.02.2021 08:30

Mathematics, 22.02.2021 08:30

Mathematics, 22.02.2021 08:30

History, 22.02.2021 08:30

Mathematics, 22.02.2021 08:30

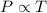 (At constant volume and number of moles)
(At constant volume and number of moles)

