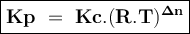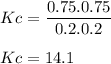Chemistry, 01.08.2019 16:30 afloareiandrei8615
An equilibrium mixture contains 0.750 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00 l container. co(g)+h2o(g)↽−−⇀co2(g)+h2(g) how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol once equilibrium has been reestablished?

Answers: 1


Other questions on the subject: Chemistry




You know the right answer?
An equilibrium mixture contains 0.750 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions in other subjects:

Spanish, 19.04.2021 06:20


Mathematics, 19.04.2021 06:20

Biology, 19.04.2021 06:20


Mathematics, 19.04.2021 06:20




![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/0158/5375/9d1db.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/0158/5375/b3cf6.png)