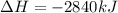Chemistry, 01.08.2019 16:30 carlalopezelox2244
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), according to the equation below. mc020-1.jpg the enthalpy of the reaction is –2,840 kj. what is the heat of combustion, per mole, of glucose? –2,840 kj/mol –473.3 kj/mol 473.3 kj/mol 2,840 kj/mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 21.06.2019 22:30, elizabethprasad2
How many grams of n2h4 will be consumed by 23 g of n2o4
Answers: 1

Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3
You know the right answer?
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), acco...
Questions in other subjects:





Mathematics, 14.06.2021 18:50

Social Studies, 14.06.2021 18:50

History, 14.06.2021 18:50


Mathematics, 14.06.2021 18:50

History, 14.06.2021 18:50