

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1


Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
In the following combustion reaction, what is the mole ratio of diatomic oxygen to water? 2 c2h6 + 7...
Questions in other subjects:



Chemistry, 10.12.2020 01:20




World Languages, 10.12.2020 01:20

Geography, 10.12.2020 01:20


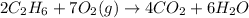
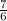 .
.

