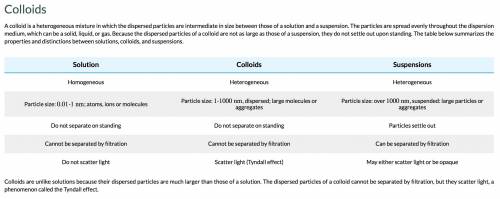
Consider a mixture of soil and water and compare it to a colloid, such as milk. which property best differentiates these two mixtures? soil and water is a suspension because it consists of minute particles suspended in the medium. milk is a colloid because it consists of larger particles suspended in the medium, which start to settle when allowed to stand. soil and water is a colloid because it consists of minute particles suspended in the medium. milk is a suspension because it consists of larger particles suspended in the medium, which start to settle when allowed to stand. soil and water is a colloid because it has a uniform composition. milk is a suspension because it doesn’t have a uniform composition. soil and water is a suspension because it consists of larger particles suspended in the medium, which start to settle when allowed to stand. milk is a colloid because it consists of minute particles that remain suspended in the medium. soil and water is a suspension because it has a uniform composition. milk is a colloid because it doesn’t have a uniform composition.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2


Chemistry, 22.06.2019 03:40, allyyzz
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1
You know the right answer?
Consider a mixture of soil and water and compare it to a colloid, such as milk. which property best...
Questions in other subjects:

Mathematics, 26.06.2019 21:00





Physics, 26.06.2019 21:00

Biology, 26.06.2019 21:00