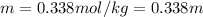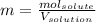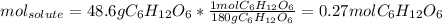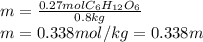Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Asolution contains 48.6 g glucose (c6h12o6) dissolved in 0.800 l of water. what is the molality of t...
Questions in other subjects:

Chemistry, 27.04.2021 18:30

Mathematics, 27.04.2021 18:30



Mathematics, 27.04.2021 18:30





Mathematics, 27.04.2021 18:30