
Chemistry, 02.08.2019 10:30 lokaranjan5736
What mass of ammonia is necessary to react with 2.1x10^24 molecules of oxygen in 4nh3+7o2=6h2o+4no2 * show steps*

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
What mass of ammonia is necessary to react with 2.1x10^24 molecules of oxygen in 4nh3+7o2=6h2o+4no2...
Questions in other subjects:





Physics, 24.09.2019 02:00




Mathematics, 24.09.2019 02:00

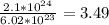 moles of oxygen (O2). By the stoicheiometry of the reaction, for every 7 moles of oxygen reacting, we need 4 moles of ammonia. Hence, we need 4/7*3.49 moles = 2 moles of ammonia. We need to calculate its weight; we know that the atomic mass of hydrogen is 1 and that the atomic mass of nitrogen is 14 (atomic and molecular masses are measured in grams). Hence, the molecular mass of ammonia (that contains 3 hydrogen atoms and a nitrogen atom) is: 3*1+1*14= 17 gr. The molecular mass of a substance is how much one mole of molecules of this substance weighs. Hence, the 2 required moles of ammonia weigh 2*17=34 gr. 34 gr of ammonia is the necessary quantity.
moles of oxygen (O2). By the stoicheiometry of the reaction, for every 7 moles of oxygen reacting, we need 4 moles of ammonia. Hence, we need 4/7*3.49 moles = 2 moles of ammonia. We need to calculate its weight; we know that the atomic mass of hydrogen is 1 and that the atomic mass of nitrogen is 14 (atomic and molecular masses are measured in grams). Hence, the molecular mass of ammonia (that contains 3 hydrogen atoms and a nitrogen atom) is: 3*1+1*14= 17 gr. The molecular mass of a substance is how much one mole of molecules of this substance weighs. Hence, the 2 required moles of ammonia weigh 2*17=34 gr. 34 gr of ammonia is the necessary quantity.

