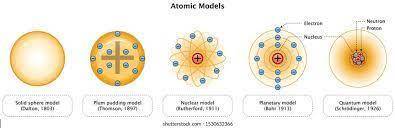
Chemistry, 02.08.2019 14:00 jslaughter3
Thomson's atomic model is best described by which of the following statements? (2 points) a nucleus with electrons moving around it like planets a positive solid sphere with electrons dispersed throughout neutrons surrounded by orbiting electrons a negative sphere with electrons surrounding it 2. which of the following is not part of dalton's atomic theory? (2 points) all matter is made up of atoms. all atoms of a given element are the same. different elements are made up of different atoms. atoms can be broken down into smaller pieces. 3. what describes the current model of the atom? (2 points) protons orbit the nucleus like planets orbit the sun. that electrons and protons move randomly around a nucleus. electrons travel as waves in the electron cloud that surrounds the nucleus. electrons orbit the nucleus like planets orbit the sun. 4. consider the work of thomson, rutherford, and bohr. in your opinion, which was the most important and why? be sure to state what your selected person discovered and how it contributed to the model of the atom. (4 points)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Thomson's atomic model is best described by which of the following statements? (2 points) a nucleus...
Questions in other subjects:

English, 22.01.2021 21:40

Spanish, 22.01.2021 21:40


Social Studies, 22.01.2021 21:40



Mathematics, 22.01.2021 21:40



Chemistry, 22.01.2021 21:40