
Chemistry, 11.11.2019 14:31 wowwowumbsi
Ejercicios sobre análisis gravimétrico y análisis volumétrico.
1. determine la concentración molar de disolución de naoh que
se requieren para neutralizar 0.546 g de khp si se gastaron
23.4 ml de naoh
2. calcule la constante de equilibrio para el siguiente caso: se
prepara 1l de una solución que contiene 3.00 moles de ácido
benzoico (c6h5cooh), 6.00 moles de etanol (c2h5oh) y agua.
cuando se obtiene el equilibrio se producen 1.25 moles de
benzoato de etilo (c6h5cooc2h5), como se muestra en la
siguiente reacción:
ayuda con procedimiento y
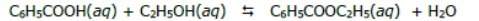

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Ejercicios sobre análisis gravimétrico y análisis volumétrico.
1. determine la concentración m...
1. determine la concentración m...
Questions in other subjects:


Mathematics, 25.08.2020 20:01





Advanced Placement (AP), 25.08.2020 20:01

Mathematics, 25.08.2020 20:01

English, 25.08.2020 20:01




