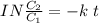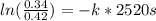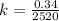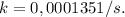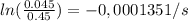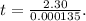Chemistry, 02.08.2019 15:30 unknown54321
It takes 42.0 min for the concentration of a reactant in a first–order reaction to drop from 0.45 m to 0.32 m at 25°c. how long will it take for the reaction to be 90% complete?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
It takes 42.0 min for the concentration of a reactant in a first–order reaction to drop from 0.45 m...
Questions in other subjects:

Physics, 24.03.2021 22:40


Mathematics, 24.03.2021 22:40





Geography, 24.03.2021 22:40

Mathematics, 24.03.2021 22:40

Mathematics, 24.03.2021 22:40