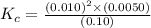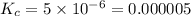Chemistry, 02.08.2019 22:30 aprilreneeclaroxob0c
For the reaction 2nobr (g) ↔ 2no (g) + br2 (g), when the concentrations are [nobr] = 0.10 m, [no] = 0.010 m, and [br2] = 0.0050 m, what is the equilibrium constant of this reaction? a. 5.0 × 10–5 m b. 0.000005 m c. 5.0 × 105 m d. 5.0 m e. none of the above

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
For the reaction 2nobr (g) ↔ 2no (g) + br2 (g), when the concentrations are [nobr] = 0.10 m, [no] =...
Questions in other subjects:







Biology, 19.02.2021 20:00

Mathematics, 19.02.2021 20:00


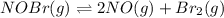
![K_c=\frac{[NO]^2[Br_2]}{[NOBr]}](/tpl/images/0163/3611/32da8.png)