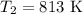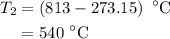Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 03:00, kdcloyd3362
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
The rate constant of a reaction is 1.1 × 10-4 s-1 at 470 °c, and the activation energy is 264 kj/mol...
Questions in other subjects:

Mathematics, 27.11.2019 08:31

Social Studies, 27.11.2019 08:31

Health, 27.11.2019 08:31


Mathematics, 27.11.2019 08:31



Mathematics, 27.11.2019 08:31

English, 27.11.2019 08:31

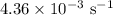 comes out to be
comes out to be  .
.
 …… (1)
…… (1)
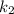 is rate constant at temperature
is rate constant at temperature  .
.
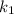 is rate constant temperature
is rate constant temperature 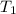 .
.
 is activation energy.
is activation energy.
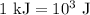

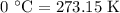

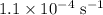 .
.