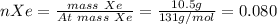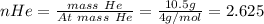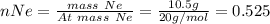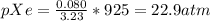Chemistry, 03.08.2019 03:30 pinapunapula
In a mixture of he, ne, and xe gases with a total pressure of 925 atm, if there is 10.5 g of each gas in the mixture, what is the partial pressure of xe?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 00:50, kaseywright3418
Which statement would indicate the presence of an acid
Answers: 3


Chemistry, 23.06.2019 10:30, erikloza12pdidtx
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
In a mixture of he, ne, and xe gases with a total pressure of 925 atm, if there is 10.5 g of each ga...
Questions in other subjects:

Mathematics, 29.03.2020 02:37


Biology, 29.03.2020 02:38





Mathematics, 29.03.2020 02:38


Mathematics, 29.03.2020 02:38

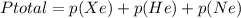 -------------(1)
-------------(1)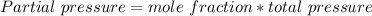
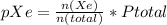 -----(2)
-----(2)