

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 07:00, kotetravels10
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1


You know the right answer?
What is the volume in ml of a 1.11 carat diamond, given the density of diamond is 3.51 g/ml? (1 car...
Questions in other subjects:



German, 30.10.2020 18:10

Mathematics, 30.10.2020 18:10

Mathematics, 30.10.2020 18:10

Business, 30.10.2020 18:10




 , where ρ is the density, m is the mass and V is the volume.
, where ρ is the density, m is the mass and V is the volume. 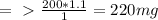
 => V =
=> V =  =
= 


