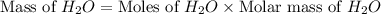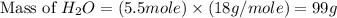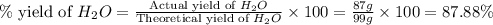Chemistry, 03.08.2019 17:00 akitchen10
Consider the chemical equation. 2h2 + o2 mc022-1.jpg 2h2o what is the percent yield of h2o if 87.0 g of h2o is produced by combining 95.0 g of o2 and 11.0 g of h2? use mc022-2.jpg.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Consider the chemical equation. 2h2 + o2 mc022-1.jpg 2h2o what is the percent yield of h2o if 87.0 g...
Questions in other subjects:




Mathematics, 07.04.2021 19:10

Mathematics, 07.04.2021 19:10


History, 07.04.2021 19:10


Mathematics, 07.04.2021 19:10

Mathematics, 07.04.2021 19:10

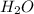 is, 87.88%
is, 87.88%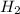 and
and 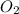 .
.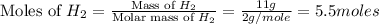
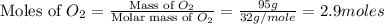
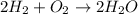
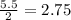 moles of
moles of 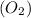 = 2.9 - 2.75 = 0.15 moles
= 2.9 - 2.75 = 0.15 moles