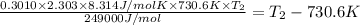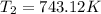Chemistry, 04.08.2019 02:00 natalieoppelt
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 hcl the activation energy is 249 kj/mol and the frequency factor is 1.6 × 1014 s−1. find the temperature at which the rate of the reaction would be twice as fast as when the reaction runs at 730.6 k. enter your answer numerically and in terms of kelvin.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2



Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 hcl the activation energy...
Questions in other subjects:



Mathematics, 16.04.2021 23:50


Computers and Technology, 16.04.2021 23:50


Computers and Technology, 16.04.2021 23:50



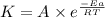
![\log \frac{K_2}{K_1}=\frac{E_a}{2.303R}\times [\frac{T_2-T_1}{T_1T_2}]](/tpl/images/0167/7789/e0c66.png)
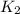 = rate of reaction at
= rate of reaction at 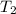
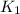 = rate of reaction at
= rate of reaction at 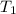
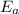 = activation energy
= activation energy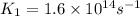
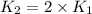
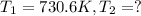
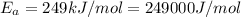
![\log \frac{2K_1}{K_1}=\frac{249000 kJ/mol}{2.303\times 8.314 J/mol K}\times [\frac{T_2-730.6 K}{730.6 K\times T_2}]](/tpl/images/0167/7789/638fd.png)
![0.3010=\frac{249000 J/mol}{2.303\times 8.314 J/mol K}\times [\frac{T_2-730.6 K}{730.6 K\times T_2}]](/tpl/images/0167/7789/3fa9a.png)