Chemistry, 04.08.2019 03:30 queenkendra16
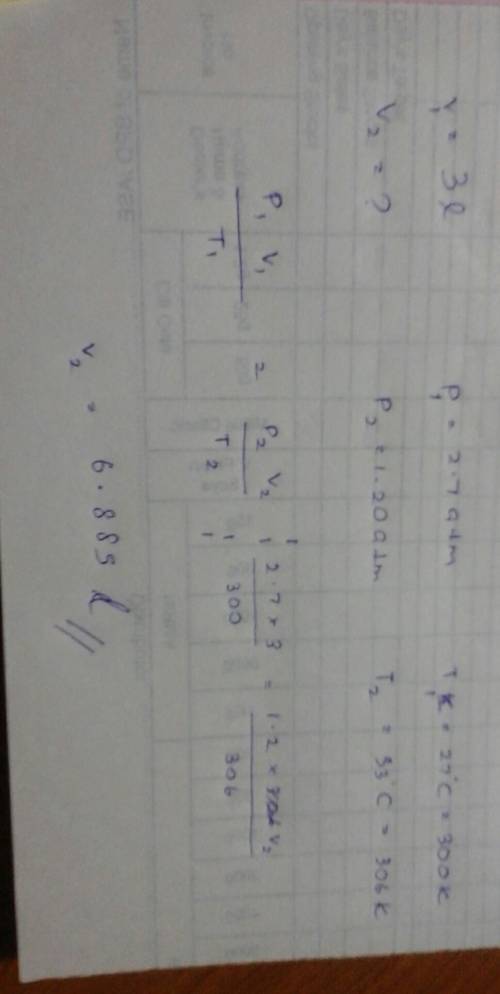
An ideal gas occupies a volume of 3.00 l at 2.70 atm and 27°c. if the temperature is raised to 33°c and the pressure is decreased to 1.20 atm, what is the final volume of the gas?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, leannamat2106
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
An ideal gas occupies a volume of 3.00 l at 2.70 atm and 27°c. if the temperature is raised to 33°c...
Questions in other subjects:

Spanish, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20

History, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20