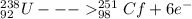proton produces nuclide x and a neutron. what is nuclide x?

Chemistry, 25.08.2019 01:30 lathwkuster
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
answers:
magnesium-24
magnesium-23
neon-23
s
odium-24
none of the above
the isotope p
has a half-life of 14.3 days. if a sample originally contained 1.00 g of p,
how much was left after 43 days?
answers:
0.250 g
0.125 g
0.750 g
0.500 g
identify x in the reaction
below.
u
+ c
→ cf
+ x
answers:
1
alpha particle
3
protons
6
neutrons
6
electrons
a .20 gram sample of c-14 was allowed to decay for 3 half-lives. what mass
of the sample will remain? carbon-14 has a half life of 5730
years.
answers:
0.025
0.05
0.1
0
.01
0.05
the isotope cu
has a half-life of 30 s. if a sample originally contained 48 mg of cu,
how much time passed before the amount fell to 3 mg?
answers:
120 s
240s
30 s
60 s(when i did my calculation for the question above, i got 60 seconds)
what radionuclide decays to fe-56
by beta emission?
answers:
fe
co
mn
co
mnhow do i know wat it becomes. i put 57 co 27 and it's wrong.
the cf
to cf
conversion is accompanied by
answers:
an alpha
emission
a
neutron capture
an electron
capture
an electron releasei would greatly appreciate your . you.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
proton produces nuclide x and a neutron. what is nuclide x?
Questions in other subjects: