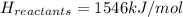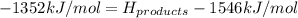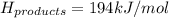Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants is 1546 kj/mol. calculate the total bonding energy of the products and decide whether the reaction is endothermic or exothermic a)84 kj/mol, endothermic b)194 kj/mol, endothermic c) 84 kj/mol, exothermic d)194 kj/mol, exothermic

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1


You know the right answer?
Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants...
Questions in other subjects:




History, 23.06.2019 03:10

Mathematics, 23.06.2019 03:10

Mathematics, 23.06.2019 03:10




Mathematics, 23.06.2019 03:10

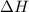 for the reaction comes out to be negative.
for the reaction comes out to be negative.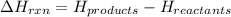
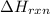 = 1352kJ/mol[/tex]
= 1352kJ/mol[/tex]