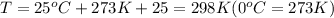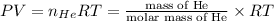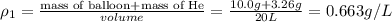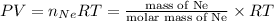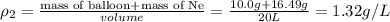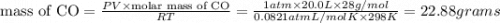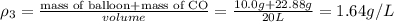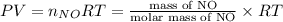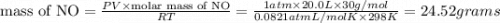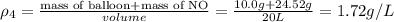Chemistry, 04.08.2019 22:10 makaylashrout77
Four balloons, each with a mass of 10.0 g, are inflated to a volume of 20.0 l, each with a different gas: helium, neon, carbon monoxide, or nitrogen monoxide. if the temperature is 25.0°c and the atmospheric pressure is1.00 atm, what is the density of each filled balloon? (note: the density of the filled balloon refers to both the contents of the balloon and the balloon itself.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 00:00, annsmith66
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Four balloons, each with a mass of 10.0 g, are inflated to a volume of 20.0 l, each with a different...
Questions in other subjects:







Mathematics, 21.02.2020 21:53