
Chemistry, 04.08.2019 16:00 NeonPlaySword
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm o2=32 g/mol; mm fe2o3=159.70 g/mol) if 63.98 g of oxygen gas is completely consumed, how many moles of iron (iii) oxide are formed? a. 1.333 mol b. 3071 mol c. 2.999 mol d. 6812 mol

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, daigle18383
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm...
Questions in other subjects:

Mathematics, 04.12.2020 21:50



Mathematics, 04.12.2020 21:50



Mathematics, 04.12.2020 21:50



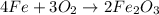
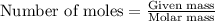
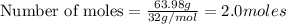
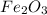 .
.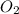 produces 2 moles of
produces 2 moles of 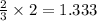 moles of
moles of 



