
The electronic configuration of an element is given below. 1s22s22p63s1 which statement about the reactivity of the element is true? it is reactive because it has a full outermost energy level. it is unreactive because it has a full outermost energy level. it is reactive because it has to lose one electron to have a full outermost energy level. it is unreactive because it has to gain one electron to have a full outermost energy level.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
The electronic configuration of an element is given below. 1s22s22p63s1 which statement about the re...
Questions in other subjects:

Mathematics, 21.01.2020 01:31




Chemistry, 21.01.2020 01:31


Mathematics, 21.01.2020 01:31

Health, 21.01.2020 01:31

English, 21.01.2020 01:31

Mathematics, 21.01.2020 01:31

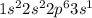 shows that there is only one valence electron present in the outermost shell.
shows that there is only one valence electron present in the outermost shell.

