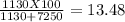Making homemade ice cream is one of life's great pleasures. fresh milk and cream, sugar, and flavorings are churned in a bucket suspended in an ice–water mixture, the freezing point of which has been lowered by adding rock salt. one manufacturer of home ice cream freezers recommends adding 2.50 lb (1130 g) of rock salt (nacl) to 16.0 lb of ice (7250 g) in a 4-qt freezer. calculate the weight percent of nacl of the solution that will result when this mixture melts.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
You know the right answer?
Making homemade ice cream is one of life's great pleasures. fresh milk and cream, sugar, and flavori...
Questions in other subjects:









Mathematics, 06.06.2020 21:59