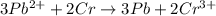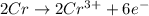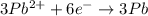Given the balanced ionic equation:
3pb2+ + 2cr ==> 3pb + 2cr3+
what is the number o...

Chemistry, 20.08.2019 11:30 mathman783
Given the balanced ionic equation:
3pb2+ + 2cr ==> 3pb + 2cr3+
what is the number of moles of electrons gained by 3.0 moles of lead ions?
(1) 5.0 mol (3)3.0 mol
(2) 2.0 mol (4) 6.0mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Questions in other subjects:






History, 02.02.2021 03:30



Mathematics, 02.02.2021 03:30