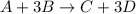Chemistry, 02.08.2019 00:30 callmedarthvadorplz
Look at the following hypothetical reaction: a+3b--> c+3d assume that the equation is balanced. in which of the following reactant mole ratios will reactant b be the limiting reagent? a) a: 4b b) a: 5b c) 2a: 7b d) a: b

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Look at the following hypothetical reaction: a+3b--> c+3d assume that the equation is balanced....
Questions in other subjects:

Social Studies, 06.12.2019 23:31

History, 06.12.2019 23:31

Mathematics, 06.12.2019 23:31

Mathematics, 06.12.2019 23:31

English, 06.12.2019 23:31

Social Studies, 06.12.2019 23:31

Chemistry, 06.12.2019 23:31