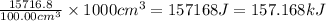Chemistry, 01.08.2019 08:20 tintlemax6256
Astudent added 5.350 g of ammonium chloride to 100.00 cm3 of water. the initial temperature of the water was 25.55℃ but it decreased to 21.79℃. calculate the enthalpy change that would occur when 1 mol of the solute is added to 1.0000 dm3 of water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Astudent added 5.350 g of ammonium chloride to 100.00 cm3 of water. the initial temperature of the w...
Questions in other subjects:


Mathematics, 06.01.2021 01:40


Chemistry, 06.01.2021 01:40



English, 06.01.2021 01:40

Biology, 06.01.2021 01:40


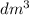 of water is 157.168 kJ/mol.
of water is 157.168 kJ/mol.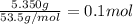
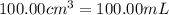
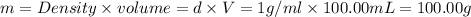
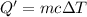
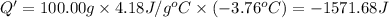
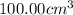 of water .
of water .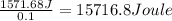
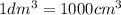
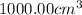 of water :
of water :