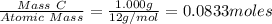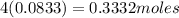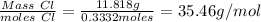Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, lil3114
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
You know the right answer?
Carbon tetrachloride contains one carbon and four chlorine atoms. for this compound 11.818 g of chlo...
Questions in other subjects:


Physics, 03.02.2020 10:44

Geography, 03.02.2020 10:44



French, 03.02.2020 10:44


Mathematics, 03.02.2020 10:44

Mathematics, 03.02.2020 10:45

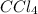 contains one carbon (C) and for chlorine (Cl) atoms
contains one carbon (C) and for chlorine (Cl) atoms