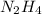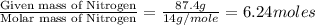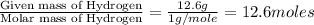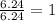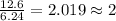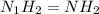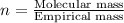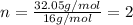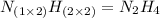Chemistry, 31.07.2019 15:10 jphelps19992019
An analysis of a compound showed it contained 87.4% nitrogen and 12.6% hydrogen. the molecular weight was determined to be 32.05 g/mol. what is the molecular formula for this compound?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1


Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
An analysis of a compound showed it contained 87.4% nitrogen and 12.6% hydrogen. the molecular weigh...
Questions in other subjects:



English, 19.07.2019 10:00



Mathematics, 19.07.2019 10:00

History, 19.07.2019 10:00

Mathematics, 19.07.2019 10:00


Mathematics, 19.07.2019 10:00