
Chemistry, 29.07.2019 19:00 elitehairnerd1964
Which of the following best explains the polarity of water (h2o)? a. protons are more attracted to the hydrogen atoms, so the hydrogen atoms become positively charged. b. since the oxygen atom has more electrons than the hydrogen atoms, it is negatively charged. c. electrons are more attracted to the oxygen atom, so there is a partial negative charge on the oxygen atom. d. since there are two positive hydrogen atoms and only one negative oxygen atom, the water molecule is positively charged.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 23.06.2019 05:00, shealynh52
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
Which of the following best explains the polarity of water (h2o)? a. protons are more a...
Questions in other subjects:





English, 06.06.2020 23:59





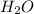 . This shows that there are two hydrogen atoms and one oxygen atom.
. This shows that there are two hydrogen atoms and one oxygen atom. 

