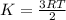Decreasing the temperature of a reaction decreases the reaction rate because a) decreasing the temperature also decreases the pressure. b) decreasing the temperature may cause a reactant to solidify. c) decreasing the temperature decreases the concentration of reactants. d) decreasing the temperature lowers the average kinetic energy of the reactants.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Decreasing the temperature of a reaction decreases the reaction rate because a) decreasing the tempe...
Questions in other subjects:

Mathematics, 16.01.2021 01:00

Mathematics, 16.01.2021 01:00

History, 16.01.2021 01:00


Social Studies, 16.01.2021 01:00

Mathematics, 16.01.2021 01:00




Mathematics, 16.01.2021 01:00