
Chemistry, 27.07.2019 17:40 Shamplo8817
Which statement correctly describes the ka of a weak acid? the ka is large, and the equilibrium favors products. the ka is large, and the equilibrium favors reactants. the ka is small, and the equilibrium favors products. the ka is small, and the equilibrium favors reactants.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3


You know the right answer?
Which statement correctly describes the ka of a weak acid? the ka is large, and the equilibrium fav...
Questions in other subjects:

Mathematics, 27.05.2021 18:10

Biology, 27.05.2021 18:10

English, 27.05.2021 18:10

Mathematics, 27.05.2021 18:10


Mathematics, 27.05.2021 18:10


Physics, 27.05.2021 18:10

Mathematics, 27.05.2021 18:10

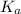 is small, and the equilibrium favors reactants.
is small, and the equilibrium favors reactants.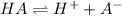
![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0757/8147/66f51.png)
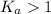 ; the reaction is product favored.When
; the reaction is product favored.When 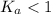 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 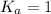 ; the reaction is in equilibrium.
; the reaction is in equilibrium.

