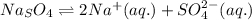Chemistry, 26.07.2019 16:20 brandydailey24pe8r24
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfate is added because? 1)the addition of sulfate ions shifts equilibrium to the left. 2)the addition of sodium ions shifts equilibrium to the left. 3)the addition of sulfate ions shifts equilibrium to the right. 4)the addition of sodium ions shifts equilibrium to the right.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

You know the right answer?
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfate is added because?...
Questions in other subjects:







Chemistry, 01.10.2019 17:10