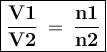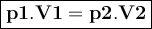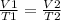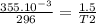Chemistry, 25.07.2019 12:40 joseflores10205
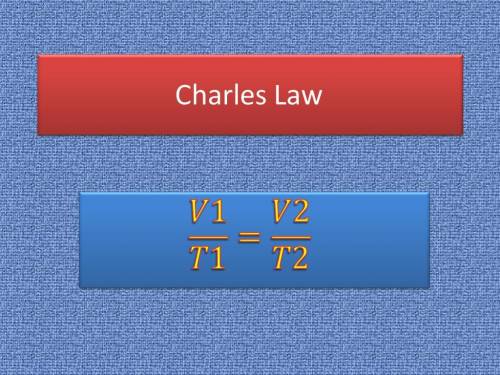
Asample of helium gas occupies 355ml at 23°c. if the container the he is in is expanded to 1.50 l at constant pressure, what is the final temperature for the he at this new volume?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Asample of helium gas occupies 355ml at 23°c. if the container the he is in is expanded to 1.50 l at...
Questions in other subjects:

Mathematics, 14.07.2020 20:01

Spanish, 14.07.2020 20:01



Mathematics, 14.07.2020 20:01

History, 14.07.2020 20:01