
Chemistry, 25.07.2019 02:00 JocelynC7237
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentration of oh− ions.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:50, lildestinyquintana
Ase your answer to this question on the information below. hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant. the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen. chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product. nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentrati...
Questions in other subjects:

English, 06.04.2021 21:40



Arts, 06.04.2021 21:40

Mathematics, 06.04.2021 21:40



Biology, 06.04.2021 21:40


Mathematics, 06.04.2021 21:40

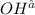 is 0.8 M.
is 0.8 M.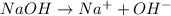


![[OH^-]](/tpl/images/0129/3993/b2910.png)


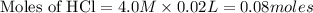
![[H^+]](/tpl/images/0129/3993/07acb.png)

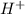 will react with 0.08 moles of
will react with 0.08 moles of 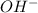 and (0.16-0.08)= 0.08 moles of
and (0.16-0.08)= 0.08 moles of ![[OH^]-=\frac{moles}{\text {Volume in L}}=\frac{0.08}{0.1}=0.8M](/tpl/images/0129/3993/83da4.png)


