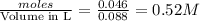Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1
You know the right answer?
28 ml of 0.10 m hcl is added to 60 ml of 0.10 m sr(oh)2. determine the concentration of oh− in the r...
Questions in other subjects:

Mathematics, 20.03.2020 13:05




English, 20.03.2020 13:05


Geography, 20.03.2020 13:05

Mathematics, 20.03.2020 13:05

Biology, 20.03.2020 13:05

Mathematics, 20.03.2020 13:05

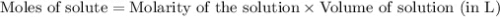 .....(1)
.....(1)
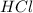 solution = 0.10 M
solution = 0.10 M

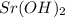 solution = 0.10 M
solution = 0.10 M
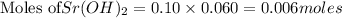
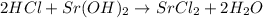
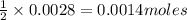 of
of ![[OH^-]](/tpl/images/0125/0620/b2910.png) will be =
will be =