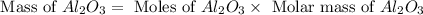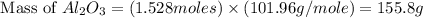Chemistry, 23.07.2019 13:20 paranoidbih
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4al(s) + 3o2(g) ® 2al2o3(s) [balanced] a mixture of 82.49 g of aluminum ( = 26.98 g/mol) and 117.65 g of oxygen ( = 32.00 g/mol) is allowed to react. what mass of aluminum oxide ( = 101.96 g/mol) can be formed?

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts...
Questions in other subjects:

Physics, 01.12.2020 01:10

History, 01.12.2020 01:10


Mathematics, 01.12.2020 01:10





History, 01.12.2020 01:10

English, 01.12.2020 01:10

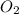 = 117.65 g
= 117.65 g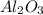 = 101.96 g/mole
= 101.96 g/mole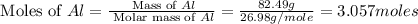
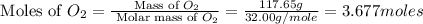
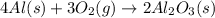
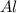 react with 3 mole of
react with 3 mole of 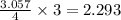 moles of
moles of 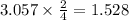 moles of
moles of