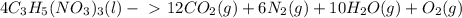Chemistry, 23.07.2019 11:00 awdadaddda
In the following reaction, how many grams of nitroglycerin c3h5(no3)3 will decompose to give 120 grams of water? 4c3h5(no3)3(l) 12co2(g) + 6n2(g) + 10h2o(g) + o2(g) the molar mass of nitroglycerin is 227.0995 grams and that of water is 18.0158 grams. 1260.3 grams 3781.67 grams 23.8 grams 3.8 grams 605.07 grams

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
In the following reaction, how many grams of nitroglycerin c3h5(no3)3 will decompose to give 120 gra...
Questions in other subjects:


Mathematics, 03.05.2021 04:20



Mathematics, 03.05.2021 04:20

Mathematics, 03.05.2021 04:20

Mathematics, 03.05.2021 04:20